Compliance. Retention. Reliability.
Two way communication between researchers & participants
Two way communication between researchers & participants
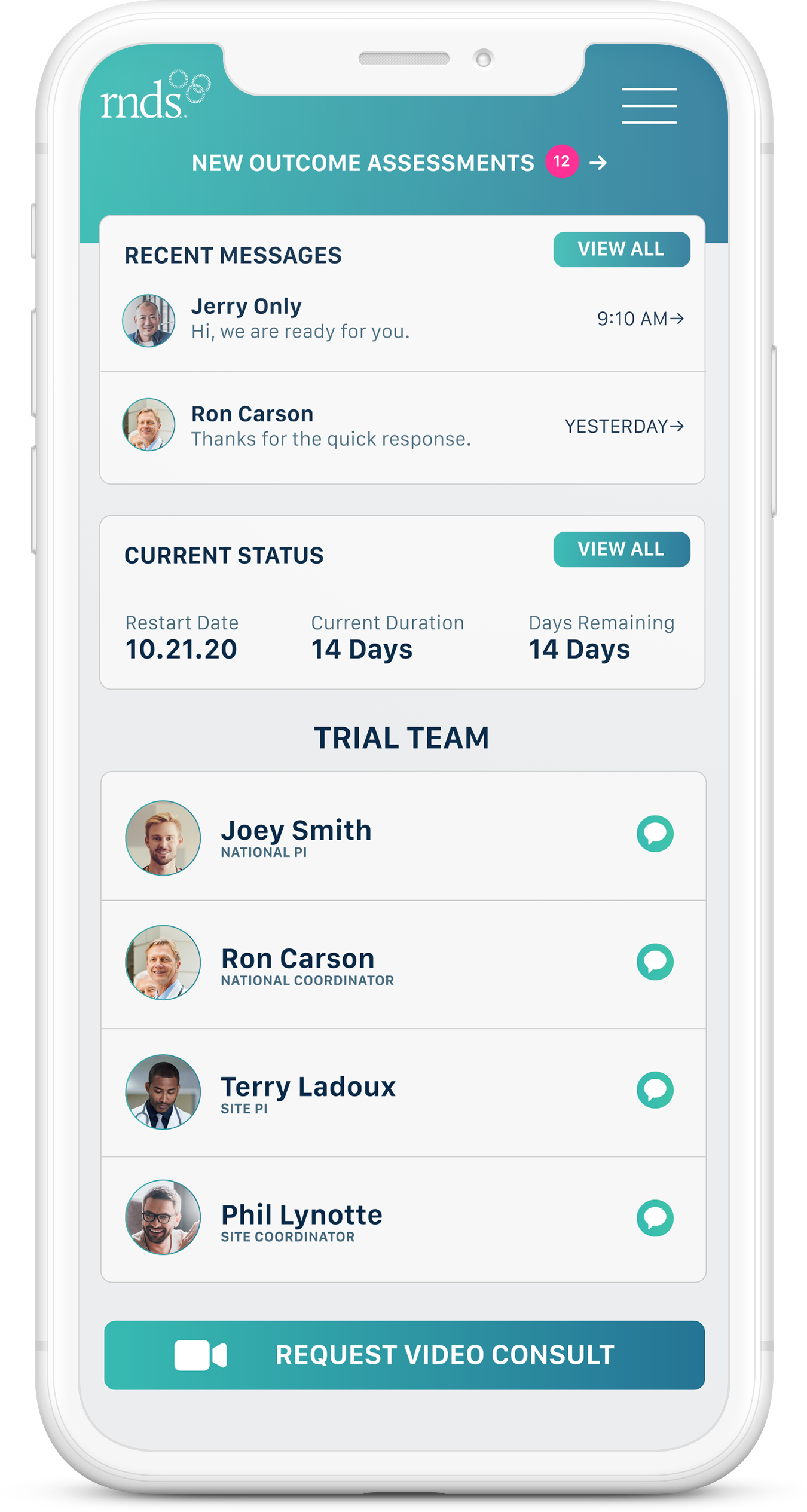
Trial Participant Dashboard
RNDS Clinical Trials is a customizable, HIPAA-compliant, dual-focused, mobile application connecting clinical providers and trial participants to efficiently collect, distribute, and display study-specific information.
RNDS Clinical Trials was created by medical & research professionals
Created by professionals who wanted to efficiently maximize their time spent with other providers and clinical trial participants, RNDS Clinical Trials allows staff-to-staff and participant-to-staff communication via text, VOIP in-app calling and real-time video consults.
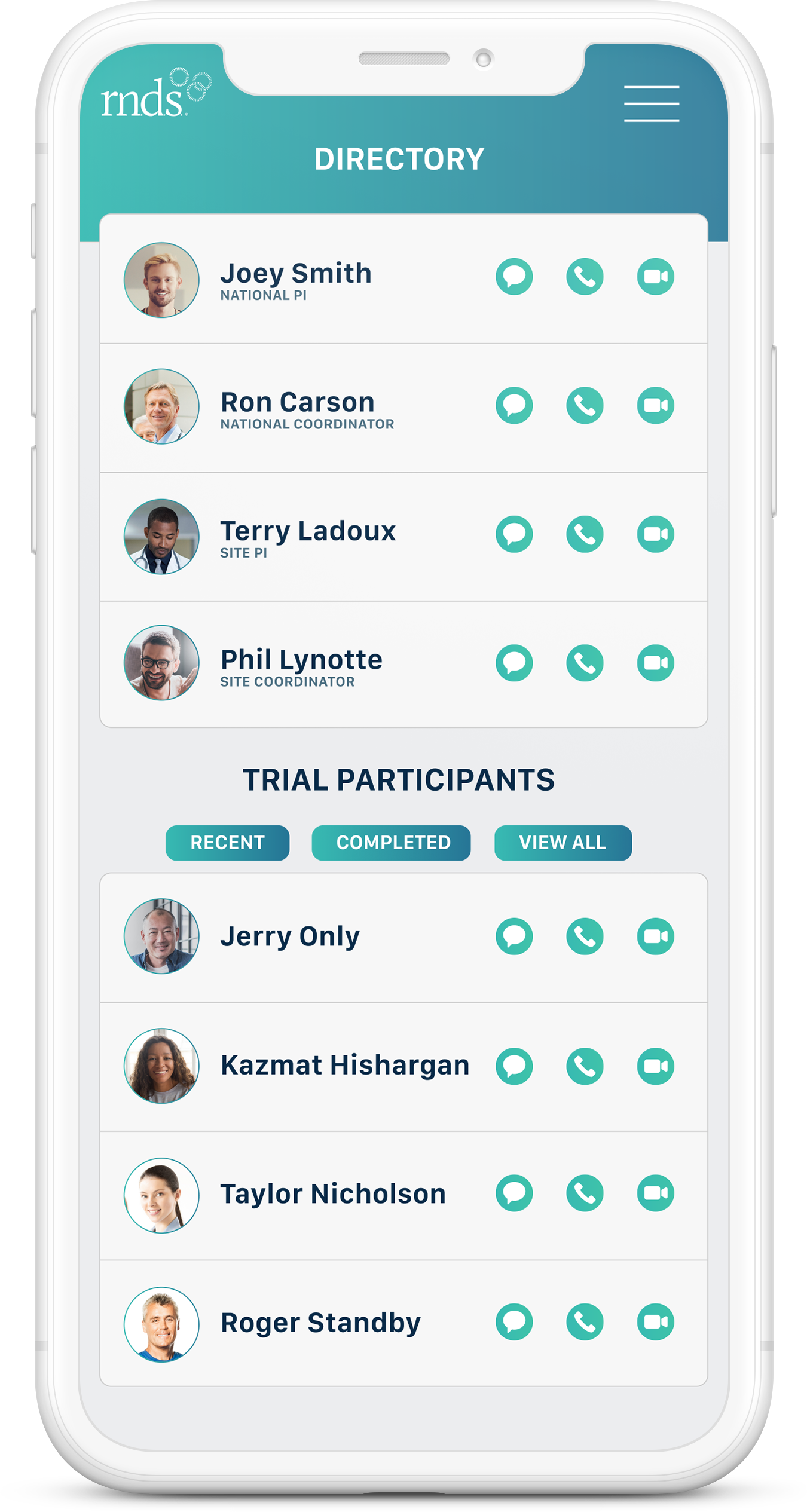
PI Dashboard
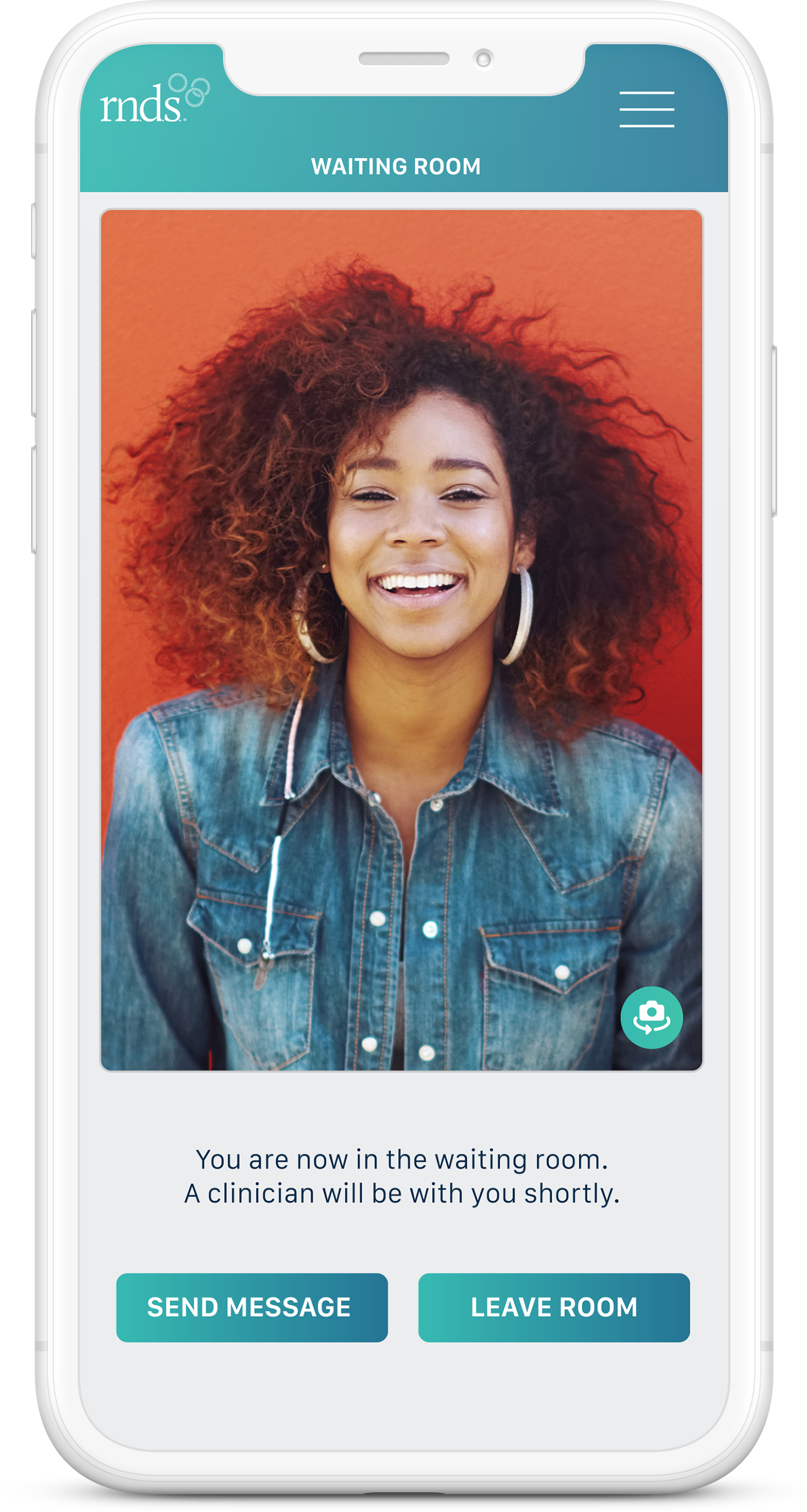
Waiting Room

Video Consult
Convenience and efficiency in one easy-to-use app
RNDS Clinical Trials digitally marries the availability schedule to the actual communication tool, eliminating the extra step of having to find out who is on call. With one button, participants and staff members can access the right trial staff member, all on a HIPAA secure cloud. Our platform also serves as a digital repository of trial-related information such as photos, consent forms, and even participant related health assessments, acting as a trial-specific, multi-platform convertible digital record.

HIPAA Secure
Analytics
Mobile Oriented
Fully Customizable
Send us a message to discuss how RNDS Clinical Trials can help you.
Contact
Tesia Holley, Senior Product Manager
for more information: